As real-world data (RWD) continues to be increasingly used by the pharmaceutical industry and encouraged by the FDA, we are exploring its value throughout the product life cycle in a four-part blog series. We already discussed how RWD can accelerate the path to regulatory approval before clinical trials even begin. All while informing study design, aiding in site selection, discovering and recruiting ideal patients and aiding enrollment screening. In this installment, we will delve into the benefits of RWD during the clinical trial.
 Prevent "lost to follow-up" patients
Prevent "lost to follow-up" patients
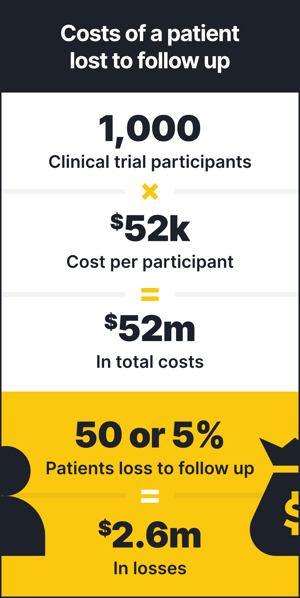
When a patient stops interacting with trial investigators, or is considered lost to follow up, it can have a serious impact on the clinical trial, leading to increased costs and potential time delays. Perhaps the single most valuable benefit to using RWD during clinical trials is that patients traditionally lost to follow up are never completely lost. If you captured the patient’s consent in advance or through disclosure in the trial IRB, you can continue to follow their healthcare journey through RWD, gaining meaningful insights such as if they have recurring diagnoses, experience potential adverse events, undergo hospitalizations or receive new treatments.
Seamlessly conduct concurrent studies
During a clinical trial, observations may be made that require further investigation or concurrent studies, such as certain populations responding differently, experiencing adverse events or developing comorbid conditions. Additionally, the treatment landscape may evolve, with changes to the standard of care. RWD allows you to rapidly respond to questions that may arise and contextualize the study, providing demographic details for participants, in-depth medical histories and potentially un-reported information that can help researchers better understand treatment responses.
Administer external control arm studies with ease
When circumstances prevent having a portion of trial participants take a placebo, whether because it is a rare disease or due to the severity of the condition, RWD can be used to design and implement a regulatory-grade external control arm.
Continual monitoring of participant healthcare utilization
During a clinical trial, you are reliant on the participant to share with you any health-related events they may experience outside of the trial, such as seeing another doctor, starting a new therapy or being treated in the emergency room. Patient’s don’t always share this information, however, whether because they’re embarrassed, fear they’ll be excused from the study, simply forget or consider it irrelevant. But not having this insight could lead to a critical gap in understanding the safety and efficacy of the drug being studied. RWD offers researchers a reliable means to monitor the healthcare utilization of participants, potentially even further demonstrating the benefits, safety and/or mitigated risks of the drug being studied.
From patient identity to compliant data exchange, all in one platform
To be able to use this valuable RWD throughout the clinical trial and beyond, you need a solution that manages patient identity, governs patient permissions, and enables on-demand retrieval of both de-identified and identifiable data while remaining fully HIPAA compliant. HealthVerity provides this end-to-end solution within a 21 CFR Part-11-certified platform.
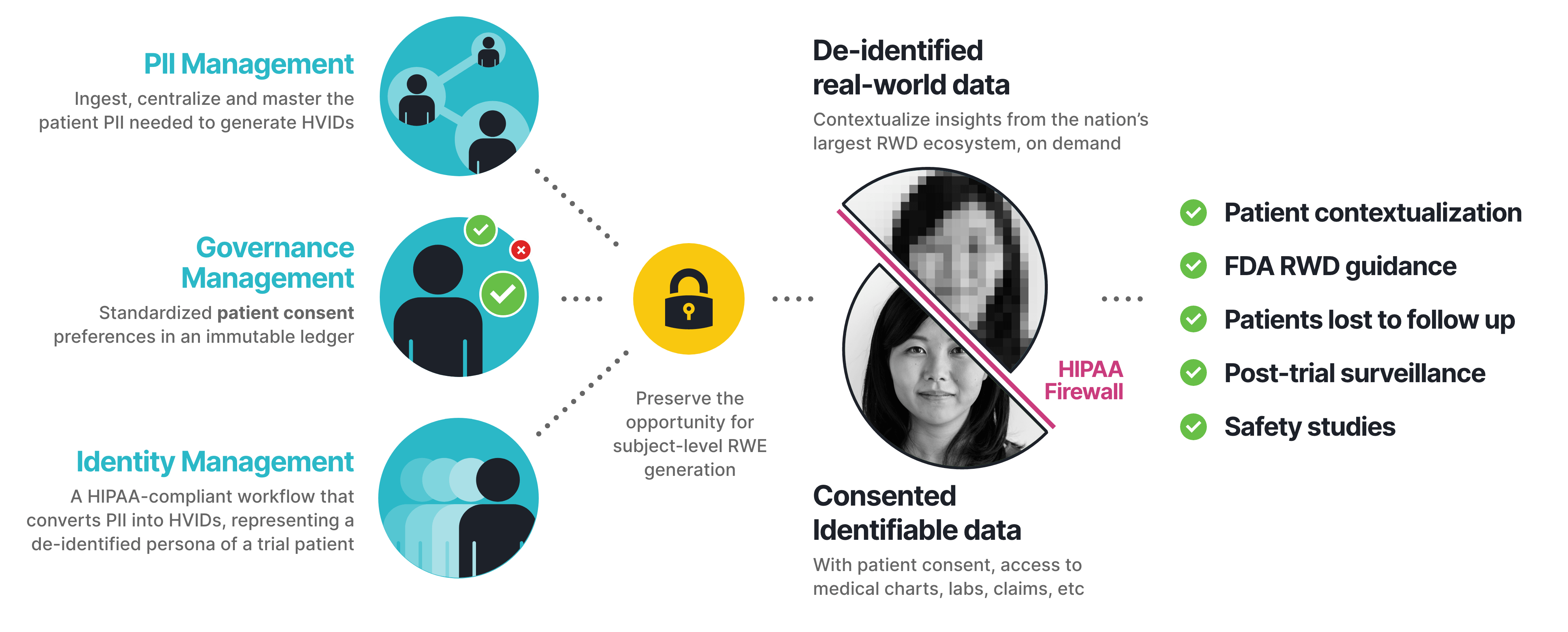
At the beginning of the trial, HealthVerity allows for ingestion of each participant’s personally identifiable information (PII), creating a centralized master that is used to generate HealthVerity IDs (HVIDs) using HealthVerity Identity Manager. This creates a privacy-protected, HIPAA-compliant single source of truth with 10x the accuracy of legacy solutions. We also capture patient consent preferences, creating an immutable ledger that lets you know what you can and cannot do with a patient’s data throughout the trial and beyond, tracking changes to preferences and all viewable from an enterprise-class dashboard. This allows for the seamless exchange of both de-identified and identifiable data from the nation’s largest healthcare and consumer data ecosystem, letting the data flow where you need it, when you need it and how you need it in a fully HIPAA-compliant manner so that you can power your analytics of choice.
In the next installment of our four-part series, we will discuss the value real-world data can provide long after the clinical trial.







