Industry trends in ulcerative colitis research: a new path
Ulcerative colitis is a chronic inflammatory bowel disease that affects about 1.5 million individuals in North America.1 Once considered a condition primarily seen in people of European ancestry, ulcerative colitis now affects a more diverse population; studies show that Hispanic individuals often have more extensive inflammation in their colon compared with European ancestry, and symptoms may present differently in Black and African-American patients.2 The disease results from auto-immune injury to the large intestine, creating a higher risk of colon cancer and symptoms such as bloody stools, abdominal cramps and urgent bowel movement.1
The therapeutic landscape has shifted quickly. Small-molecule agents, including Janus kinase (JAK) inhibitors and sphingosine-1-phosphate (S1P) receptor modulators, offer oral options with short half-lives and no risk of anti-drug antibodies.3 Tofacitinib was the first approved oral JAK inhibitor for moderate/severe UC, and other agents in this class have shown meaningful remission rates.3 S1P modulators such as ozanimod and etrasimod have also demonstrated higher clinical response and endoscopic remission than placebo.3 Meanwhile, interleukin-23 (IL-23) inhibitors such as risankizumab have produced higher remission rates at weeks 12 and 52 in phase 3 trials, leading to approval for moderate/severe disease.4
Real-world data plays an important role in studying how ulcerative colitis presents and responds to treatment outside controlled trials. A cross-sectional study linking survey data with medical and pharmacy claims included 687 patients (347 with ulcerative colitis) and showed that those with moderate or severe symptoms reported lower quality of life, greater productivity loss and higher healthcare costs than those with mild disease.5 Studies of diverse populations also reveal that African and Hispanic patients may have lower rectal inflammation but more arthritis and experience delays in diagnosis because of limited access to specialists,6 underscoring the need for real-world evidence to clarify how treatments perform across different subgroups and lines of therapy.
Across epidemiology, medical affairs and RWE functions, researchers are asking important questions:
- How do advanced therapies perform in everyday practice? Teams want to compare biologics, JAK inhibitors, S1P modulators and IL-23 antibodies on outcomes such as steroid-free remission, hospitalization, surgery and cost of care.
- Which patients are receiving these therapies, and when? Stakeholders need to understand real-world treatment patterns, escalation timing and differences by age, disease severity, race and comorbidities.
- Where do disparities persist? Many groups are focused on identifying gaps in diagnosis, specialist access and treatment response among Black and African-American, Hispanic and other underserved populations.
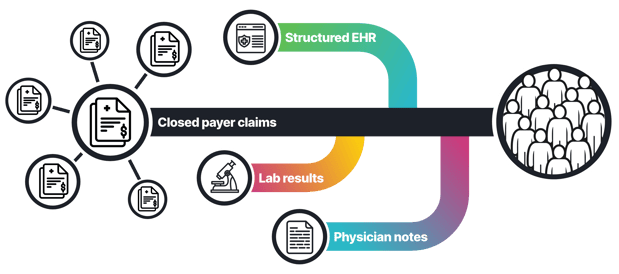
Figure 1: taXonomy Pathways can combine data from diverse sources like claims, labs, EHR including physician notes to support a variety of RWE analyses.
Current real-world evidence in ulcerative colitis and remaining gaps
Despite advances in therapy, researchers and clinicians face major barriers when working with real‑world ulcerative colitis data:
- Real-world outcomes are unclear. Moderate or severe ulcerative colitis is linked to lower quality of life, higher work loss and higher healthcare costs,5 but most datasets do not capture symptom severity or steroid free remission in a consistent way.
- Treatment use is hard to follow. Patients move between 5 ASA, steroids, biologics, JAK inhibitors, S1P modulators and IL 23 antibodies, but real care settings rarely record why therapy was started, escalated or switched, limiting the ability to study true response and failure patterns.
- Disease activity is poorly captured. Measures such as fecal calprotectin, C-reactive protein and endoscopy results are incomplete or missing in many EHR systems, making it difficult to classify mild, moderate or severe disease.
- Inequities persist. Black and Hispanic patients may have lower rectal inflammation but more arthritis and may experience delayed diagnosis because of limited access to specialists,6 yet most real-world datasets cannot quantify these gaps.
- Integration is the barrier. Claims alone cannot show severity, symptoms or response. EHR alone cannot show full medication history or cost. Linking both with labs and notes is required to understand how therapies perform across real lines of care (Figure 2).
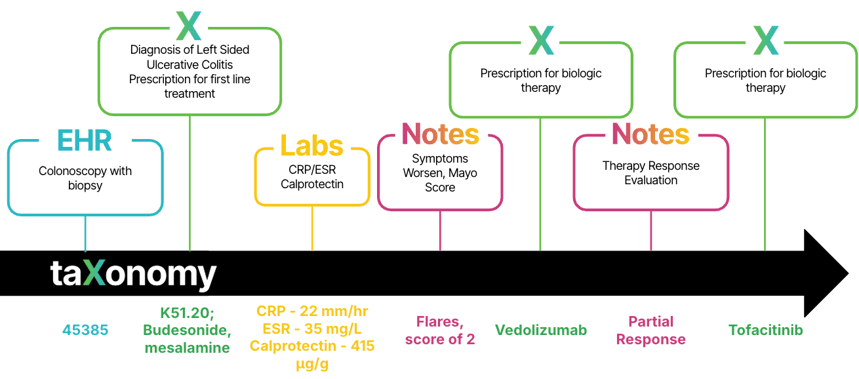
Figure 2: A comprehensive view of the ulcerative colitis patient journey captured in claims, lab and EHR data including physician notes.
Using taXonomy Pathways to build a unified dataset for the ulcerative colitis patient journey
The complex data landscape creates an opportunity for taXonomy Pathways (Figure 2). By organizing ulcerative colitis information into a consistent, research ready structure, taXonomy Pathways can align data from diverse sources and support many RWE analyses. Applied to ulcerative colitis, it can:
- Harmonize diverse data sources. taXonomy Pathways can connect EHR, claims, registries, patient-reported outcomes and demographic variables. The linkage used in the real-world data study shows that patient-reported symptom severity can be validated against claims.
- Standardize disease activity and phenotypes. It can define proctitis, left-sided colitis and pancolitis, along with mild, moderate and severe disease, enabling consistent cohort identification and comparison across datasets.
- Clarify real-world treatment pathways. By mapping biologics, small molecule therapies and IL-23 antibodies to classes and dosing schedules, taXonomy Pathways makes it possible to evaluate real-world response, switching patterns and remission benchmarks such as those seen in the risankizumab trials.
- Support safety and effectiveness surveillance. Mapping drugs such as tofacitinib, upadacitinib, ozanimod, etrasimod and risankizumab into one ontology enables continuous monitoring of real-world safety, durability and off-label use.
- Quantify economic and humanistic burden. Linking symptom severity, treatment patterns and healthcare costs allows measurement of work loss and financial burden, which is especially relevant for patients with moderate or severe ulcerative colitis.
Toward patient‑centric and equitable ulcerative colitis care
Ulcerative colitis is lived through symptoms, flares and uncertainty, not billing codes. Claims alone cannot show bleeding, pain or the reasons a therapy was stopped; those details live in labs, endoscopy and physician notes. taXonomy Pathways unites these sources into a complete, privacy-protected patient journey so researchers can study steroid free remission, mucosal healing, safety and long term effectiveness with accuracy.
Behind every data point is a person trying to avoid hospitalization, manage a flare or return to their daily life. HealthVerity is committed to supporting research that honors those patients by giving teams the evidence they need to understand real world treatment pathways and inequities. taXonomy Pathways helps ensure that decisions in ulcerative colitis are grounded in truth, transparency and the full reality of the patient journey.
To get started with taXonomy Pathways: Schedule a meeting with an expert; watch a replay of our taxonomy Pathways webinar, or explore the solution below.
References
- Voelker R. What is ulcerative colitis? JAMA. 2024;331(8):716. doi:10.1001/jama.2023.23814
- Barnes EL, Loftus EV, Kappelman MD. Effects of race and ethnicity on diagnosis and management of inflammatory bowel diseases. Gastroenterology. 2021;160(3):677-689. doi:10.1053/j.gastro.2020.08.064
- Yao D, Ran Z. New progress of small-molecule drugs in the treatment of inflammatory bowel disease. Chinese Medical Journal. 2024;137(5):556. doi:10.1097/CM9.0000000000003026
- Louis E, Schreiber S, Panaccione R, et al. Risankizumab for ulcerative colitis: two randomized clinical trials. JAMA. 2024;332(11):881-897. doi:10.1001/jama.2024.12414
- Naegeli AN, Balkaran BL, Shan M, Hunter TM, Lee LK, Jairath V. The impact of symptom severity on the humanistic and economic burden of inflammatory bowel disease: a real-world data linkage study. Current Medical Research and Opinion. 2022;38(4):541-551. doi:10.1080/03007995.2022.2043655
- Florence-Damilola O, Aboubakr A, Anyane-Yeboa A. Inflammatory bowel disease in underserved populations: lessons for practice. Current opinion in gastroenterology. 2022;38(4):321. doi:10.1097/MOG.0000000000000855