In case you missed it, at the end of August, the FDA released new guidance on how to approach using real-world data (RWD). It’s important to note because the guidelines are meant to accelerate outcomes and speed development of novel medical products. As evidence, FDA Commissioner Robert Califf stated at the Medical Device Innovation Consortium last month, “I believe we’re moving very rapidly now to a place where more and more of the data we use for evidence is going to come from the so-called real-world clinical environment.”1
Today, RWD generated outside of clinical trials, from sources such as labs, pharmacy, electronic health records, insurance claims and more are largely kept independent from the data generated by clinical trials. These new FDA guidelines are meant to address that separation, providing recommendations for incorporating RWD into clinical trial designs to support regulatory decision making.
There are three specific pain points that we are going to highlight that benefit from incorporating technology, RWD and the new guidance:
- Long-term follow up - The struggle to follow clinical trial participants after a clinical trial
- Lost to follow up - The difficulty when clinical trial participants no longer communicate or visit with the investigator
- Reimbursement - The challenge in getting reimbursement for key drugs, particularly those in rare disease or gene therapies
#1 - In for the long haul
While drugs and therapies often show strong benefits and efficacy over the short term, we are unable to confirm if the drug results in adverse events that were not identified during the clinical trial. Per the guidance, the FDA would like to better understand when adverse events occur and recognizes the potential of RWD to monitor these outcomes over the long term and well beyond the end of trial. Today, however, most clinical trials run their course without capturing the additional safety signals that could be gained from RWD to better understand the impact of the drug or therapy.
Pharmaceutical companies need new approaches to harness the insight from RWD while following the new FDA guidance. With HealthVerity FLOW, a modularized technology solution that synchronizes patients across de-identified and identifiable data in a fully governed, HIPAA-compliant and 21 CFR Part 11-certified environment (which is also recommended in the guidelines), we aim to solve the problem of unrealized insights.
HealthVerity FLOW delivers this capability with three elements:
- First, clinical trial participants are de-identified and receive a universal identifier, known as a HealthVerity ID (HVID), which serves as a single source of truth that allows you to easily discover their RWD.
- Next, consent and user permissions for both identifiable and de-identified data are captured and managed, along with data usage rights to ensure full HIPAA compliance.
- Lastly, HealthVerity FLOW synchronizes the clinical trial participant with both de-identified and identifiable RWD using the HVID — and keeping the two separate — for the seamless discovery and exchange of a near limitless combination of research-ready, HIPAA-compliant RWD.
#2 - What was lost can be found
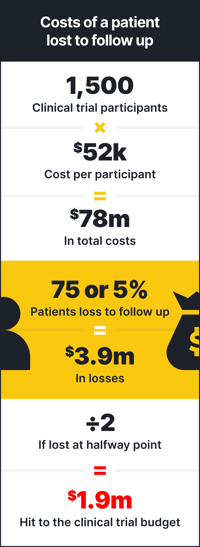 Participants lost to follow up is a daunting proposition for every clinical trial. Let’s break down the cost of a patient lost to follow up:
Participants lost to follow up is a daunting proposition for every clinical trial. Let’s break down the cost of a patient lost to follow up:
Per the FDA, the average phase 3 clinical trial requires between 300 and 3,000 participants.2 While the average cost per participant varies based on therapeutic area and other factors, let’s assume an average cost of $52,000 per patient. With those numbers, if we assume a phase 3 clinical trial with 1,500 patients at the cost of $52,000 each, that’s $78 million. Research suggests patients are lost to follow up at a rate between 5% and 20%.3 If we assume that the lost to follow up rate is on the lower end at 5%, that’s 75 patients. The cost of those 75 patients then is $3.9 million. But there was data collected on those patients up until they dropped out, so, let’s assume they dropped out at the halfway point. That’s still a $1.95 million hit to the clinical trial budget.
Now, more recruitment has to occur to enroll patients and the clinical trial duration is at risk of being prolonged. The patients that were traditionally lost to follow up, however, are not completely lost with HealthVerity FLOW. Since the patient may have provided consent to capture their RWD, those patients can still provide meaningful data about their health and wellness through their RWD and the $1.95 million is not completely lost.
#3 - Demonstrating value
When a drug proves effective, gains regulatory approval and reaches market, the cost of the drug is often prohibitive for those who need it the most. With the patients from the clinical trial assigned an HVID, you have the ability to follow their long-term healthcare journey and compare the cost of their care while taking the drug to those not receiving the treatment. This could provide meaningful insights that demonstrate how taking the drug ultimately drives down the total cost of care when compared to the additional healthcare resources needed to control the condition for those not getting the drug.
The green light for accelerating drug approval
According to the FDA, only 25-30% of phase 3 clinical trials reach a new drug application.2 With the new guidelines the FDA is giving a green light to use RWD to generate the real-world evidence needed to improve this outlook and accelerate the development of drugs for the people who need them. There are safeguards, however, to ensure data privacy and integrity. By synchronizing the foundational elements of Identify, Privacy, Governance and Exchange, which are at the core of HealthVerity FLOW, we meet those guidelines, providing unparalleled accuracy in identity resolution and patient matching, built-in Privacy compliance, and Governance of data usage rights and permissions for the Exchange of the RWD you need for the most comprehensive view of the patient journey. Real-world data and clinical trial data no longer have to be disparate and siloed. It can be synchronized with HealthVerity FLOW to accelerate outcomes.
1Al-Faruque, Ferdous (September 22, 2023). FDA’s Califf: Expect to see more RWE-based regulatory decisions. Regulatory Focus. https://www.raps.org/News-and-Articles/News-Articles/2023/9/FDA%E2%80%99s-Califf-Expect-to-see-more-RWE-based-regulato.
2U.S. Food and Drug Administration (FDA). Step 3: Clinical Research.https://www.fda.gov/patients/drug-development-process/step-3-clinical-research#:~:text=Researchers%20design%20Phase%203%20studies,involve%20300%20to%203%2C000%20participants.
3Dettori, J.R. (2011). Loss to follow-up. Evidence-Based Spine-Care Journal. February 2011; 2(1): 7-10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3427970/.







