October is Breast Cancer Awareness Month. While pink ribbons and fundraising campaigns help bring attention to the disease, the latest data show that breast cancer remains a pressing public health issue. In 2025, about 1 in 8 women in the U.S. will develop breast cancer during their lifetime.1 An estimated 316,950 women and 2,800 men will be diagnosed with invasive breast cancer this year, with another 59,080 diagnosed with ductal carcinoma in situ. Mortality rates have declined by roughly 44% since 1989. thanks to earlier detection and improved therapies, yet disparities persist. Black women, for example, have a breast‑cancer incidence rate slightly lower than white women but a 38% higher mortality rate.1 Thanks to earlier detection and improved treatments, more than 4 million survivors now live in the United States, yet breast cancer remains the second-leading cause of cancer death among women.1
That mix of progress and persistence demonstrates why it’s critical to understand each patient’s full experience, starting from screening and diagnosis to treatment and survivorship. The details of a patient’s care pathway reveal where interventions succeed and where disparities remain, particularly among populations more likely to experience aggressive disease or delayed diagnosis.
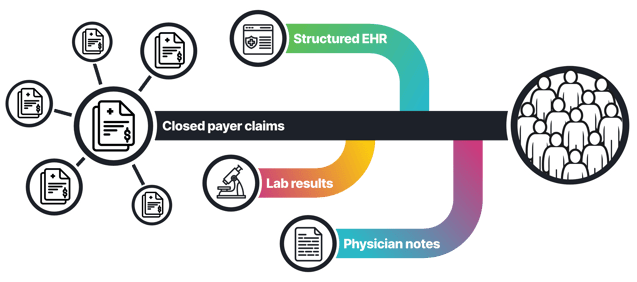
Through taXonomy Pathways, we enable researchers and life-science organizations to build a comprehensive picture of the patient journey. By unifying real-world data (RWD) across closed claims, electronic health records (EHR), lab results, biomarkers, imaging and even physician notes, HealthVerity helps uncover the clinical context that traditional data aggregators and closed claims alone often miss. These synchronized insights enable scientists and public-health experts to study how and why breast cancer evolves, how treatments perform, and where gaps in access or equity persist, all with the precision and outstanding privacy safeguards that define the HealthVerity data ecosystem.
Beyond statistics: why the patient journey matters
National campaigns often focus on raising awareness, but seeing patients only as numbers hides the complexity of their experiences. Survivorship is not only about avoiding death; it includes quality of life, mental health and financial impact. To develop treatments that improve outcomes and reduce inequities, researchers need a detailed view of the patient journey from preventive care through diagnosis, treatment and long‑term follow‑up.
Real‑world data (RWD) provide that comprehensive view. By combining information from medical claims, electronic health records (EHR), laboratory results, pharmacy claims and even imaging and physician notes, analysts can observe how patients move through the healthcare system. RWD can help:
- Identify eligible trial participants. Linked claims and EHR data reveal patients with specific tumor subtypes, genetic mutations or comorbid conditions.
- Monitor patients during studies. Continuously updating claims and lab records offer insights into healthcare utilization and adherence outside of the trial setting.
- Create control arms. RWD enables concurrent or external control arms for clinical trials, reducing the need for placebo groups.
- Conduct long‑term follow‑up. Longitudinal data help researchers evaluate real‑world safety and effectiveness years after a product enters the market.
Patient story: following a breast cancer patient in real-time
To illustrate how comprehensive data reveal the patient journey, consider Gina, a 34‑year‑old Black woman (represented by a de‑identified HealthVerity ID) who diligently attends her preventive appointments. Her story is hypothetical but reflects common clinical scenarios and shows how linking claims, EHR, labs and other data sources helps clinicians and researchers understand breast‑cancer pathways and outcomes.
Routine preventive care
During her annual wellness visit, claims and EHR data show that Gina’s vital signs and lab results are normal. She has a diagnosis of obesity but no family history of breast cancer, and her physical breast exam is unremarkable.
Six months later, at her annual gynecologist visit, claims and lab records indicate that she had a Pap smear and human papillomavirus (HPV) test. During the visit physician notes indicate she reports a lump and swelling in her right breast. The gynecologist documents the mass in the EHR and refers her for a mammogram.
Screening and diagnosis
Two weeks later the lab results from her gynecologic visit show a positive HPV test but a normal Pap smear. Gina visits an imaging center for a screening mammogram. The radiologist notes an abnormality and orders a breast MRI. The EHR captures these tests along with her reported symptoms.
The MRI reveals a small mass, prompting a referral to an oncologist. At the oncology consult, claims data show orders for BRCA testing, hormone‑receptor assays (estrogen receptor [ER], progesterone receptor [PR] and HER2) and an ultrasound‑guided biopsy. Because Gina is young and Black, the oncologist wants to rule out hereditary risk factors.
The genetic test results indicate a BRCA1 mutation, one of the inherited gene alterations responsible for 5–10% of breast cancers.1 A few days later, the pathology report confirms triple‑negative breast cancer (TNBC). TNBC lacks ER, PR and HER2 receptors and therefore does not respond to hormone therapy or HER2‑targeted drugs; it accounts for roughly 10% of breast cancers overall but 20% of cases in Black women.1
Treatment decisions
Gina and her oncologist discuss treatment options. Because TNBC tends to grow quickly and has a higher risk of recurrence, they decide on a mastectomy followed by radiation therapy. Claims data indicate that during a pre‑surgery follow‑up visit Gina reports symptoms of depression and anxiety. Her EHR documents and physician notes reflect this and a referral to a mental‑health specialist and initiation of an antidepressant. Pharmacy claims indicate that Gina picked up the prescription.
Surgery and post‑operative care
The EHR, claims and lab data show that Gina undergoes a mastectomy with clear margins. Post‑operative pathology confirms that the tumor was small and lymph nodes are negative.
Radiation and follow‑up
After her incision heals, Gina begins radiation therapy five days a week for six weeks. Claims and EHR records document each session. Over the next year, regular follow‑up visits include blood tests and imaging to monitor for recurrences that are captured in labs. Gina’s RWD shows no new symptoms or abnormal results. She enters remission and continues to receive periodic surveillance.
How taXonomy Pathways enriches the patient journey
taXonomy Pathways initiative expands the scope of RWD by connecting disparate datasets—closed medical and pharmacy claims, EHRs, laboratory results, imaging, repeated measures such as vital signs and physician notes—under a single, privacy‑preserving framework. Traditional claims data provide a broad view of diagnoses and healthcare utilization, but they often lack clinical detail. taXonomy Pathways fills that gap by:
- Linking claims with rich clinical data. It captures biomarker testing, genetic results, therapy selections and disease progression events from EHRs and clinician notes. In oncology, for example, researchers can observe which patients undergo BRCA testing or receive targeted therapies, not just that they were billed for an infusion.
- Tracking repeated measures over time. Longitudinal records of weight, body‑mass index, blood pressure and laboratory values allow analysts to assess treatment response and comorbid conditions in obesity and metabolic disease.
- Integrating imaging and diagnostic data. Mammograms, MRIs and other images provide context about tumor size and spread, while pathology and radiology reports add granularity.
- Maintaining patient identity resolution. The HealthVerity privacy‑preserving identity framework allows data from multiple sources to be synchronized for the same individual without revealing personal identifiers, ensuring HIPAA compliance.
By going beyond claims, taXonomy Pathways delivers a comprehensive patient journey, enabling researchers and life‑science companies to generate robust real‑world evidence. This holistic view supports health economics and outcomes research (HEOR), pharmacovigilance, clinical trial feasibility studies, and analyses of health disparities. It also empowers clinicians and advocates to design interventions that improve outcomes and equity.
References
- American Cancer Society. Breast Cancer Facts & Figures 2024-2025. Atlanta, GA: American Cancer Society; 2024. Accessed October 27, 2025. https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html






