The rise of GLP-1 therapies and the evolution of obesity treatment
Over 40% of adults in the U.S. have obesity, representing over 100 million patients.1 Not long ago, bariatric surgery was the only option for substantial weight loss among those with treatment resistant obesity. Today, a new generation of GLP-1 receptor agonists has transformed obesity and metabolic disease research and treatment. These GLP-1 drugs, including semaglutide and tirzepatide, have shown 15-25% body weight reduction in clinical trials, approaching the effectiveness of surgery. Demand for these treatments has surged and U.S. spending on GLP-1 medications rose over 500% from 2018 to 2023, climbing from $13.7 billion to $71.7 billion. Analysts project that within five years, annual spending on GLP-1 obesity drugs could exceed $100 billion.1
“With the possible exception of Operation Warp Speed, GLP-1 therapies have defined the decade in terms of impact on population health. They’re ushering in a new standard of care and a renewed push towards innovation in metabolic conditions and beyond. Still, this mechanism was discovered in the 1980s - it took us 40 years to get here. As an industry, how do we speed this up? What data or evidence do we need to get these discoveries from bench to bedside faster, while prioritizing safety, efficacy, and effectiveness?”
- Alexa Woodward, PhD, Senior Director of Strategic Accounts at HealthVerity
Nearly every major manufacturer is investing in obesity pipelines, including combination therapies that target multiple pathways. Tirzepatide’s success as a dual GLP-1/GIP agonist is leading the way for next-generation multi-receptor agonists, and companies are exploring oral formulations pills to win over patients averse to injections. Eli Lilly recently reported that its daily oral GLP-1 candidate (orforglipron) achieved significant weight loss (~12–15% over 36 weeks) with side effects similar to injectable GLP-1s.2 At the same time, there have been some setbacks. Pfizer halted development of its oral GLP-1 danuglipron in 2025 after a trial participant developed liver injury, reflecting safety concerns that require ongoing monitoring with real-world data.3 Mild to moderate gastrointestinal side effects are common for most patients on GLP-1s, prompting careful dose titration and monitoring.4
Beyond weight loss, researchers are also discovering benefits of GLP-1 drugs in other disease areas. A 2025 review in Cell highlighted the benefit of GLP-1 therapies on anti-inflammatory mechanisms involved in cardiovascular, kidney and liver conditions, and disorders like obstructive sleep apnea.5 These broad potential indications, coupled with payer concerns about affordability, access and long-term cost-effectiveness, make it clear that GLP-1 obesity treatments are more than a passing trend and they demand robust evidence and longer term real-world monitoring.
.png?width=1905&height=460&name=GLP-1%20and%20RWE%20graphic%20(1).png)
Why claims data alone can’t explain real-world GLP-1 outcomes
As adoption accelerates, pharmaceutical companies are racing to innovate through new formulations, delivery options, and combination approaches that improve tolerability and long-term outcomes. Health economists and payers want to know the true eligible population and budget impact; epidemiologists and outcomes researchers need to track long-term effectiveness and safety; pharmacovigilance experts are monitoring for adverse signals beyond clinical trials. Traditional claims data alone, however, cannot answer many of these critical questions.
Claims can reveal when a patient fills a prescription or undergoes a procedure that was billed to their insurance, but for obesity care they rarely capture the clinical details that define treatment response. In health economics and outcomes research (HEOR) and epidemiologic studies, claims data alone leave researchers without essential information such as:
- Body mass index (BMI) or weight trajectory to identify obesity thresholds or track improvement
- A1c and lipid panels to assess cardiometabolic change
- Liver and kidney markers such as ALT, AST, and eGFR to evaluate comorbidities like MASH or nephropathy
- Physician notes that document adherence barriers, recommended dosing schedules, or dietary guidance
These gaps in claims mean that researchers are missing many aspects of the patient journey (Figure 1). Claims data might show that a patient filled a 90-day prescription of Wegovy®, then stopped. Lab and electronic health record (EHR) data can reveal why. Perhaps the patient’s liver enzymes spiked, or they experienced severe nausea or their insurer refused to cover further refills.
Figure 1: A metabolic use-case comprehensive patient journey view with taXonomy Pathways
In clinical trials, GLP-1 drugs showed high adherence (over 85% of patients stayed on therapy), yet real-world use tells a different story: one large study found only 32% of obese patients remained on a GLP-1 therapy after one year, with just 27% meeting standard adherence thresholds.6 Understanding these adherence gaps and real outcomes is impossible without linking claims to the clinical evidence found in labs and EHR, including physicians notes. The problem is a data disconnect because the pharmacy and medical claims tell us that GLP-1 usage is soaring, but they don’t tell us which patients benefit, which patients fail, and why.
A recent AMA/CDC report covered the challenges with long term adherence and the introduction of new indications will drive the need for ongoing research incorporating richer data sources beyond claims.7
The research opportunity connecting claims with real-world data
The opportunity for manufacturers, researchers, and healthcare stakeholders is to bridge this data gap and gain a comprehensive view of the GLP-1 obesity treatment journey. By integrating claims with clinical data, researchers can find insights that were previously hidden. This is particularly valuable for teams in health economics and outcomes research (HEOR), epidemiology, and pharmacovigilance who are tasked with evaluating real-world performance.
With the right data:
- HEOR analysts can model the true impact of GLP-1 therapies – for example, linking the 15%-20% weight loss achieved by patients to downstream reductions in comorbidity and healthcare utilization.8
- Epidemiologists can better estimate the size of the eligible population (using BMI and lab criteria instead of diagnosis codes) and understand how treatment patterns evolve over years.
- Pharmacovigilance teams can proactively detect adverse events or off-label use trends by observing lab anomalies (like liver enzyme elevations) or reading physician notes that mention events like pancreatitis or gallbladder issues which might not trigger an insurance claim code.
- Market access and medical affairs teams to demonstrate the full value of these therapies. Real-world evidence can show payers which subpopulations benefit most and inform more equitable coverage policies.
The end result is a chance to maximize the health benefits of GLP-1 obesity treatments (which have enormous potential) while containing risks and costs through data-driven insights.
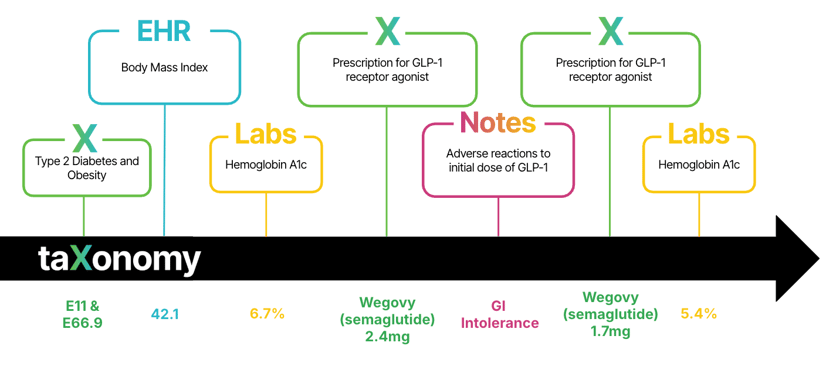
Figure 2: the diabetes and obesity patient journey with taXonomy Pathways.
The taXonomy Pathways solution
taXonomy Pathways brings closed payer claims together with lab results and EHR, including physician notes, so you can evaluate eligibility, initiation, titration, response and persistence on therapy in one research‑ready data set.
This solution is anchored by the nation’s largest source of closed medical and pharmacy claims (covering over 300 million patients across hundreds of payers with 9+ years of history), and it adds context those claims with lab data from the two largest outpatient labs that capture over 60% of U.S. lab testing, EHR data including millions of clinical notes. The result is a data asset that allows your team to analyze the full obesity patient journey in one place, without the pain of stitching together fragmented data sources.
The dataset connects claims, labs and EHR, including physician notes to analyse:
- Who meets drug label or payer criteria based on actual BMI and comorbidities
- How titration and dosing progress in real-world care
- What metabolic outcomes patients achieve over time
- Which safety signals, such as liver or renal changes, emerge across populations
- Why patients discontinue or switch treatments, as documented in notes
See real-world GLP-1 evidence in action
Register for our webinar where we dive deeper into GLP-1 real-world evidence. In the meantime, if you are exploring obesity outcomes for therapies like Wegovy®, Zepbound® or next-generation weight loss drugs, we can help design a tailored real-world study. Tell us your population of interest and endpoints. With taXonomy Pathways, we will deliver a dataset and analysis framework that you simply cannot see in claims data alone.
Interested in getting started with taXonomy Pathways? Download our guide to comprehensive patient outcomes.
References
- Institute for Clinical and Economic Review (ICER). Affordable Access to GLP-1 Obesity Medications: Strategies to Guide Market Action and Policy Solutions. Published 2023. Accessed October 2025. https://icer.org/wp-content/uploads/2025/04/Affordable-Access-to-GLP-1-Obesity-Medications-_-ICER-White-Paper-_-04.09.2025.pdf
- Wharton, S., Aronne, L. J., Stefanski, A., Alfaris, N. F., Ciudin, A., Yokote, K., Halpern, B., Shukla, A. P., Zhou, C., Macpherson, L., Allen, S. E., Ahmad, N. N., & Klise, S. R. (2025). Orforglipron, an oral small-molecule glp-1 receptor agonist for obesity treatment. New England Journal of Medicine, NEJMoa2511774. https://doi.org/10.1056/NEJMoa2511774
- BioSpace. Pfizer ends oral GLP-1 candidate danuglipron due to liver injury. Published April 2025. Accessed October 2025. https://www.drugs.com/news/pfizer-ends-testing-obesity-pill-danuglipron-after-possible-liver-injury-124534.html
- Ghusn, W., & Hurtado, M. D. (2024). Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks. Obesity Pillars, 12, 100127. https://doi.org/10.1016/j.obpill.2024.100127
- Gonzalez-Rellan, M. J., & Drucker, D. J. (2025). The expanding benefits of GLP-1 medicines. Cell Reports. Medicine, 6(7), 102214. https://doi.org/10.1016/j.xcrm.2025.102214
- Gleason, P. P., Urick, B. Y., Marshall, L. Z., Friedlander, N., Qiu, Y., & Leslie, R. S. (2024). Real-world persistence and adherence to glucagon-like peptide-1 receptor agonists among obese commercially insured adults without diabetes. Journal of Managed Care & Specialty Pharmacy, 30(8), 860–867. https://doi.org/10.18553/jmcp.2024.23332
- Spending on GLP-1s has grown dramatically. Here are the details. (2025, August 27). American Medical Association. https://www.ama-assn.org/public-health/prevention-wellness/spending-glp-1s-has-grown-dramatically-here-are-details
- AMA passes resolution calling for expanded access to anti-obesity medications. (n.d.). Retrieved October 8, 2025, from https://www.endocrine.org/news-and-advocacy/news-room/2025/ama-passes-resolution-calling-for-expanded-access-to-anti-obesity-medications